By Kate Buron
When The U.S. Food and Drug Administration (FDA) approved the CardioMEMS HF System in 2014, Abbott harnessed the power of artificial intelligence (AI) coupled with telemedicine for managing patients with heart failure (HF). Expanded use of the system was the objective of a new trial initiated in 2018, but the onset of the COVID-19 pandemic in 2020 may have skewed the results.
AI use in HF management
AI use in health care represents a wide range of technologies that can identify and process information to solve problems that would have previously required human effort. To put it into perspective, health care-related AI applications can range from a watch that measures the wearer’s heart rate (narrow AI) to complex machine learning programs that analyze thousands of datasets in seconds (strong AI).
Because the field of cardiology is complex and rapidly changing, providers who treat patients with HF can greatly benefit from incorporating AI into their practice. Technologies to help prevent, diagnose, treat and manage patients with HF are readily accessible and can save time, improve care and outcomes, and reduce costs industrywide.
CardioMEMS for HF management
CardioMEMS HF System, Abbott’s first-of-its-kind AI sensor and processor, allows providers to remotely monitor pressure changes in the pulmonary artery (PA) that could indicate worsening HF, even before patient feels symptoms. Traditionally, clinicians have used physiological markers such as weight and blood pressure to detect worsening HF. The problem with relying on these measures is that they must be taken in person to ensure accuracy, and that by the time these signs manifest disease has often progressed significantly.
The haemodynamic-guided system is comprised of three components: a paper clip-sized, battery-free sensor that is permanently implanted in the PA during a minimally invasive procedure; a transvenous catheter to deploy the sensor; and an electronic system that processes the sensor’s signals and transfers the measurements to a secure database for analysis. Pressure changes in the PA detected by the CardioMEMS sensor are so subtle they can be identified before the patient or physician would notice any physical changes to spark concern.
The CHAMPION study and initial approval
CardioMEMS HF System was first studied for use in NYHA class III HF in the 2014 study titled, “Sustained Efficacy of Pulmonary Artery Pressure to Guide Adjustment of Chronic Heart Failure Therapy (CHAMPION),” which showed a significant reduction in rehospitalization for patients implanted with the device after being admitted with a HF-related event in the year prior.
Researchers concluded from the results of CHAMPION, conducted using a sample of 550 participants randomly assigned to receive treatment with the system (n=270) or standard care (n=280) , that use of CardioMEMS was safe and had low rates of adverse effects or malfunctions. The early PA pressure changes detected by the system’s sensor cued providers to titrate medication or adjust other treatment, often without requiring an in-person visit and leading to lower mortality and better quality of life for patients.
The FDA agreed with the study’s authors and granted St. Jude Medical (later acquired by Abbott) approval for CardioMEMS to treat class III HF in May 2014. The director of the agency’s devices sector commented that the first permanent, completely remote system providing measurements of systolic, diastolic and mean PA pressure would significantly reduce HF-related hospitalizations, potentially improving quality of life for up to 6 million patients in the U.S. living with class III HF.
What is class III HF?
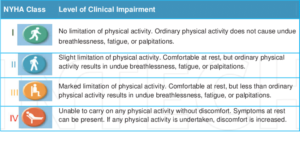
Because HF is a broad term that encompasses many symptoms, structural changes and cardiometabolic markers, New York Heart Association (NYHA) has identified four classes of HF based on patients’ limitations when engaging in physical activity and how comfortable they are at rest. The table shows the four classes of HF severity, with Class I being least severe and Class IV being most severe.
Class III HF is diagnosed when a patient shows marked limitation when performing less-than-ordinary physical activity but is comfortable at rest. Although the 2014 FDA approval of CardioMEMS for class III HF was innovative, its use in patients with class II HF could be equally important since it’s possible that interventions earlier in the course of the disease could prevent progression to classes III and IV.
The GUIDE-HF study and COVID-19 considerations
Because of its success in Class III HF patients, Abbott sought to bring CardioMEMS to a larger patient population in the “Haemodynamic-Guided Management of Heart Failure (GUIDE-HF)” study which began recruiting in 2018, before the onset of the COVID-19 pandemic. GUIDE-HF examined an expanded patient population, including patients with NYHA class II and IV HF, to evaluate the device in patients with earlier- or later-stage disease than had been studied in CHAMPION.
Investigators recruited participants with NYHA classes II-IV HF who had either been hospitalized in the last 12 months with an acute HF event or had elevated natriuretic peptide levels in the 30 days priors to enrollment. Participants were randomly assigned (1:1) to receive either therapy with CardioMEMS HF System or the usual course of care and treatment. The combined endpoints of HF-related hospitalization, urgent HF visits and mortality failed to show any statistically significant differences between the study and control groups. It was noted that when analyzed separately, the HF-related hospitalizations in the study group narrowly missed statistical significance.
It must be noted that the endpoints of the study were being measured during the timeframe that COVID-19 cases began to spike in the U.S. The study’s authors state that the COVID-19 pandemic clearly affected their outcomes and that most clinical trials conducted during this time period would require an adjusted method of statistical analysis to account for unintended factors. They proposed a prespecified impact analysis in which the trial’s primary endpoint be adjusted to March 2020, when a state of emergency was declared in the U.S., affecting patient compliance, wellbeing and overall health, all of which would impact study results. While this adjusted endpoint did not show a significant reduction in urgent HF visits or mortality, it did show a marked reduction (28%) in HF-related hospitalizations.
Opportunities for HF device development
In their August 2021 updated guidelines for the diagnosis and treatment of acute and chronic HF, the European Society of Cardiology (ESC) identified several areas where gaps exist in the medical community’s knowledge of HF diagnosis and treatment. The society indicated that devices and interventions for patients with HF is one such area of opportunity and recommended further research to deliver strong evidence for new discoveries in implantable HF devices. This gap could leave the door open for more funding and investigation into the CardioMEMS and similar systems.
Key takeaway
Although HF is still the leading cause of death worldwide, better treatments have resulted in longer lifespans for patients. Thus, the number of individuals living with HF is increasing (an estimated 6.2 million people in the U.S.) which necessitates a constant pursuit of novel devices and therapies for these patients. Wearable and implantable AI devices for HF have increased over the last few decades, providing data to improve diagnostic, triage and management techniques.
Despite unconvincing results from the GUIDE-HF trial, the CardioMEMS HF System remains a useful AI tool to help providers remotely manage patients with class III HF, reducing hospitalization and improving quality of life. The FDA’s decision to allow pre-pandemic portion of the study data to be analyzed independently – along with the ESC’s identification of knowledge gaps in this area – may provide a path for more investigation of the system in expanded patient populations.