Regeneron Pharmaceuticals, Inc. announced on March 22, 2023, that the U.S. Food and Drug Administration (FDA) had extended the approval of Evkeeza® (evinacumab) as an adjunct to other lipid-lowering therapies to treat children ages 5 to 11 with homozygous familial hypercholesterolemia (HoFH).
Regeneron’s angiopoietin-like 3 (ANGPTL3) inhibitor Evkeeza (evinacumab) was first approved by the FDA in February 2021 to treat dangerously high cholesterol in addition to other lipid-lowering therapies in patients 12 years and older with HoFH. Because HoFH results in high levels of low-density lipoprotein cholesterol (LDL-C) very early in childhood and has few treatment options, the expanded use of Evkeeza in patients younger than 12 has been long-awaited news for families, providers, and health advocacy organizations such as the Family Heart Foundation.
“At the Family Heart Foundation (FHF), we know that children with homozygous familial hypercholesterolemia, and those caring for them, often live in fear of what the future holds as they contend with the dangerously high levels of bad cholesterol, or LDL-C, caused by this genetic disorder,” “Only 5% of rare diseases actually have an FDA-approved treatment. With this FDA approval, the HoFH community now has a much-needed treatment for young children, potentially making it possible for many to achieve recommended LDL-C levels much earlier in the course of this rare disease. This is a hopeful development for those living with HoFH.” – Mary McGowan, MD, FHF Chief Medical Officer
In their announcement of Evkeeza’s expanded indication, Regeneron executives called attention to the pivotal trial that led to the approval in patients with HoFH as young as 5 years old. Despite already using lipid-lowering therapies, children who entered the trial had an average LDL-C level of 264 mg/dL, which is more than twice the target level of <110 mg/dL for pediatric patients with HoFH.In the first phase of the trial, 6 children with HoFH were given the drug and assessed for pharmacokinetics, safety, and tolerability. During the second stage of the study, the use of evinacumab over the course of 24 weeks in 14 participants showed a reduction in LDL-C levels by 48%. In fact, 79% of the participants’ LDL-C levels were reduced by half at the 24-week mark. The researchers also observed significant improvements in other key secondary endpoints, including apolipoprotein B, non-HDL-C and total cholesterol.
“Since it was first approved, Evkeeza has become the standard of care for homozygous familial hypercholesterolemia in those aged 12 years or older. We’re gratified that now children as young as 5 years old have the potential to benefit from this treatment. As a first-in-class medicine for this relentless disease, Evkeeza exemplifies the promise of genetics-based research to transform treatment paradigms. Evkeeza’s journey from target discovery to treatment innovation was only made possible due to our long-term investment in genetics research and monoclonal antibody technologies, and this remains a central tenet of our science-first approach to this day.”- George D. Yancopoulos, M.D., Ph.D., President and Chief Scientific Officer at Regeneron.
Homozygous vs. Heterozygous Familial Hypercholesterolemia
Familial hypercholesterolemia (FH) is an inherited genetic disorder characterized by high levels of LDL-C, contributing to the buildup of plaque in the arteries that can lead to cardiovascular disease, heart attack and stroke. There are two known forms of FH, each caused by mutations in one of several genes responsible for LDL-C regulation. Heterozygous FH (HeFH) is the milder form of the condition and is the result of inheriting only one mutated copy of an FH-related gene. Individuals with HeFH typically have moderately elevated LDL-C levels and are at an increased risk for heart disease. The prevalence of HeFH is estimated to be approximately 1 in 250 individuals. On the other hand, homozygous FH (HoFH) is the more severe form of FH caused by inheriting two copies of a mutated FH gene, one from each parent. Individuals with HoFH have dangerously high levels (usually >400 mg/dL) of LDL-C from birth and are at a much higher risk of developing heart disease in childhood or early adulthood. HoFH is extremely rare; the disease is estimated to affect just 1,300 people in the U.S.
Individuals with HeFH can manage their LDL-C levels with lifestyle changes and cholesterol-lowering medications such as statins, but people living with HoFH have limited treatment options and are at risk for premature atherosclerotic disease and cardiac events even in their teenage years. Until the recent expanded approval of Evkeeza, the only treatment option for patients younger than 12 years old with HoFH was LDL apheresis.
Evkeeza Considerations in Pediatric Practice
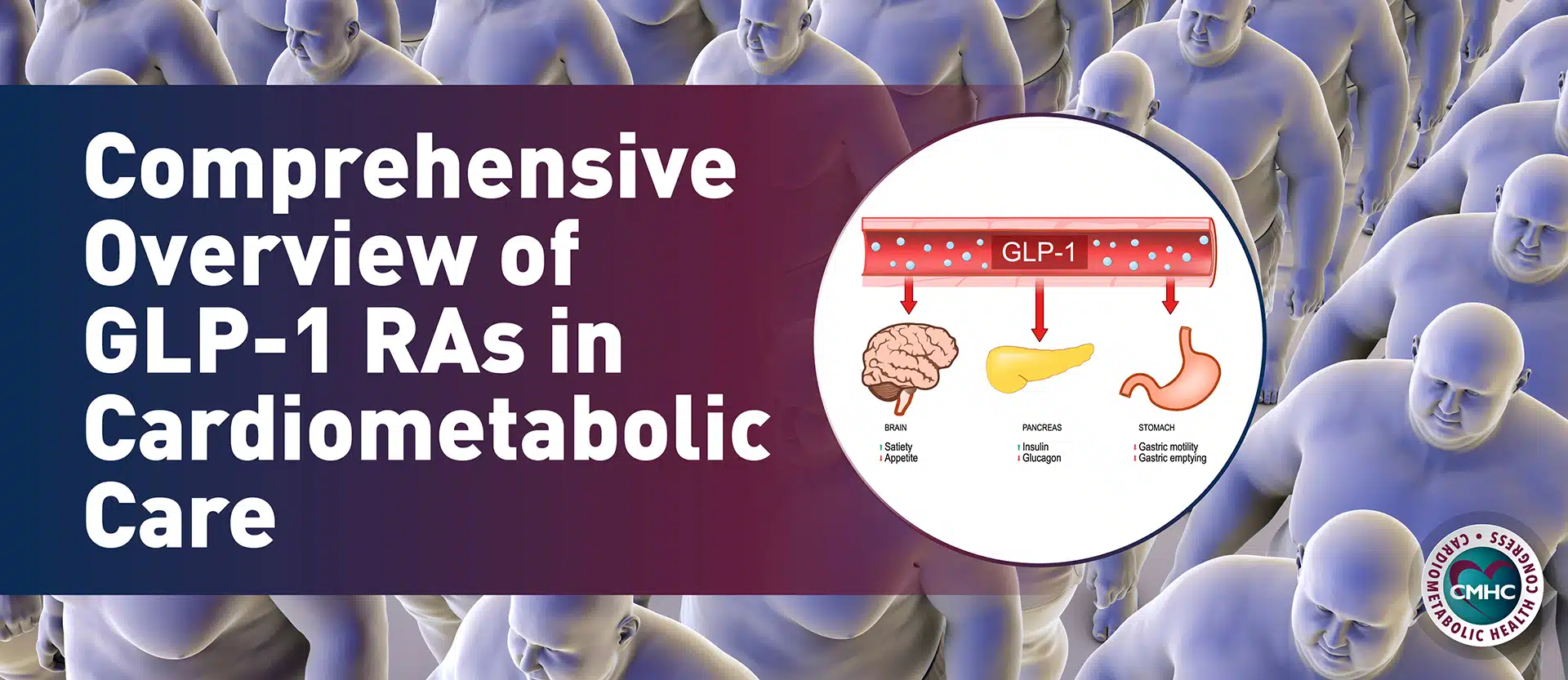
For providers who treat pediatric patients with diagnosed HoFH, the label expansion for Evkeeza may come with both optimism and practical questions for prescribing and safety. Often, just getting the HoFH diagnosis is the biggest challenge for young patients, as Carissa M. Baker-Smith, MD, MPH, co-director of Nemours Cardiac Center Cardiovascular Research and Innovation Program, knows well. “Guidelines recommend screening all children at high risk for homozygous familial hypercholesterolemia starting at age 2. However, until now, a positive diagnosis was often met with the frustration of having limited treatment options to help these children,” she said. Evinacumab, which works by blocking the PCSK9 protein, is an administered as an intravenous infusion every four weeks to help regulate cholesterol levels in the body.
Key takeaway
The approval of Evkeeza for pediatric patients with HoFH represents an important advancement in the treatment of this rare genetic condition, which can lead to cardiovascular disease, heart attack and stroke if left untreated.