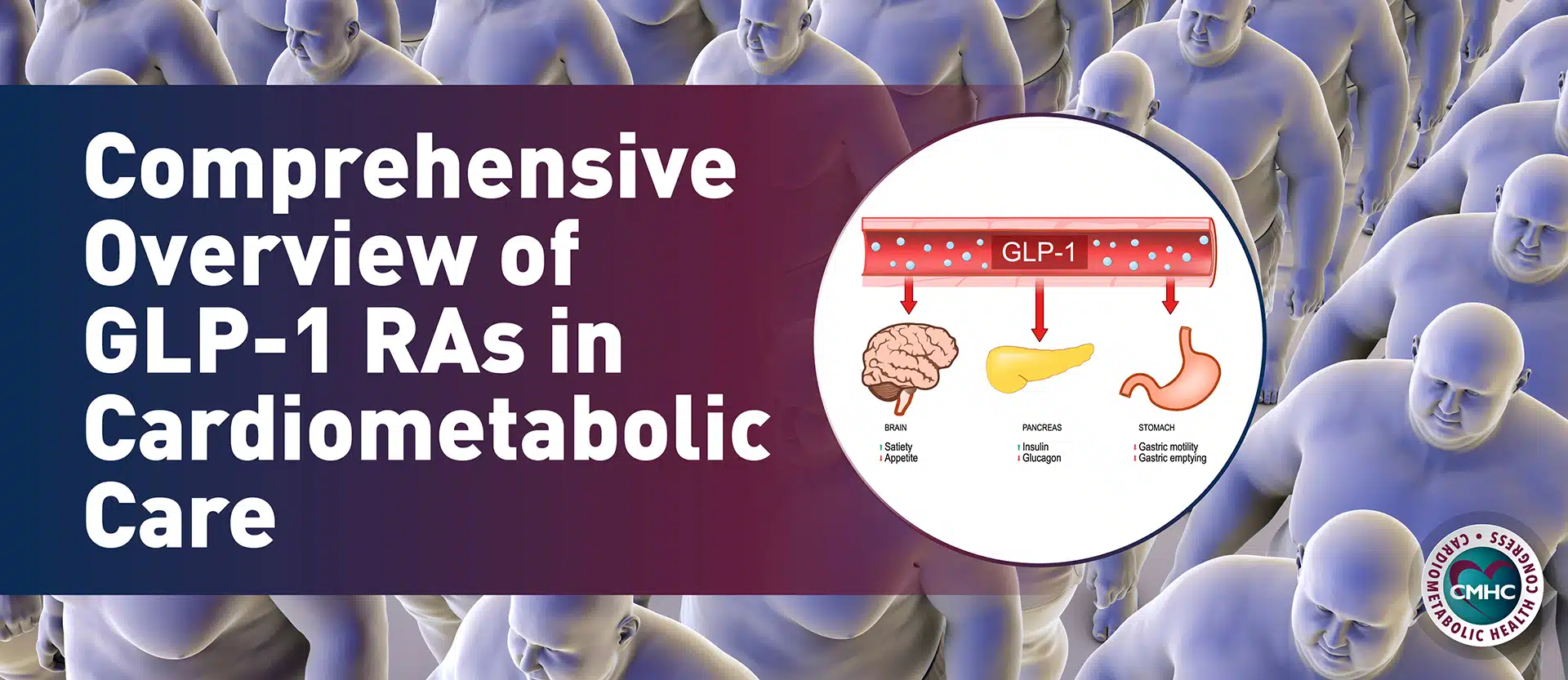
The U.S. Food and Drug Administration (FDA) announced on May 26, 2023, it had approved the once-daily oral tablet sotagliflozin to reduce the risk of cardiovascular death, hospitalization for heart failure, and urgent heart failure visit in adults with heart failure or heart type 2 diabetes, chronic kidney disease, and other cardiovascular risk factors.
Research presented at the American College of Cardiology’s 70th Annual Scientific Sessions supported the use of sotagliflozin, manufactured and distributed under the brand name Inpefa by Arkansas-based pharmaceutical company Lexicon, as the first dual sodium-glucose co-transporter type 1 (SGLT1) and sodium-glucose co-transporter type 2 (SGLT2) inhibitor for the treatment of both heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF). Sotagliflozin is the first drug in the SGLT class shown to lower glucose levels by helping the body eliminate more sugar through urination.
Clinical evidence for sotagliflozin
The FDA approval is based on two randomized, double-blind, placebo-controlled Phase 3 trials in patients with (or at risk of) heart failure:
- The SCORED trial evaluated whether sotagliflozin prevented cardiovascular events, deaths due to a heart attack or stroke, and hospitalizations or urgent visits for heart failure in patients with diabetes and chronic kidney disease. In 10,584 randomly assigned to either treatment or placebo, those who took sotagliflozin demonstrated a reduction in cardiovascular events by 26% compared with placebo after 16 months.
- The SOLOIST-WHF trial evaluated whether sotagliflozin prevented cardiovascular deaths and hospitalizations or urgent visits for heart failure in patients with diabetes and worsening heart failure. In 1,222 patients randomly assigned to treatment or placebo, those who took sotagliflozin demonstrated a reduction in cardiovascular deaths and hospitalizations or urgent visits for heart failure by 33% compared with placebo after 9 months.
Together, the SCORED and SOLOIST-WHF trials showed that sotagliflozin significantly reduced risk of hospitalizations for heart failure, urgent visits for heart failure, and cardiovascular death compared to placebo.
Guidance on the use of SGLT inhibitors
The American College of Cardiology (ACC) issued a new document in the April 2023 edition of Journal of the American College of Cardiology recommending that SGLT inhibitors be initiated in all patients with heart failure regardless of injection fraction. This guidance, entitled 2023 ACC Expert Consensus Decision Pathway on Management of Heart Failure with Preserved Ejection Fraction, states that SLGT inhibitors be administered once a patient is stable during hospitalization for a cardiovascular event as long as there are no contraindications.
Expert perspective
Cardiometabolic Health Congress (CMHC) faculty member Deepak L. Bhatt, MD, MPH, led a pooled analysis of patient data from the SCORED and SOLOIST-WHF trials, both of which published in the New England Journal of Medicine. Based on the findings Dr. Bhatt and colleagues found a “significant reduction in the primary endpoint irrespective of patients’ ejection fraction at study entry.” Dr. Bhatt went on to say, “Treatment with sotagliflozin robustly and significantly reduced cardiovascular adverse events across the full spectrum of patients with heart failure, including patients who have heart failure with preserved ejection fraction (HFpEF), for which no effective treatment is currently available. The benefit for patients with HFpEF is striking — this is the first trial to find a significant benefit in this population. We believe that these results merit a recommendation that patients who have both diabetes and HFpEF should be treated with sotagliflozin or another medication in its class.”
Sotagliflozin cost
The affordability of a new drug may cause concern among providers who’ve seen their patients unable to afford or access new medications in the past, particularly upon inital approval when they are only available as expensive brand-name drugs. Lexicon has assured the medical community it will price Inpefa “on par with existing branded heart failure medications.” For comparison, the wholesale price of Eli Lilly’s Jardiance, likely Inpefa’s main competitor, is $570.48 for a 1-month supply. In data presented from the annual AHA meeting in Boston on May 9, 2023, Washington, D.C.-based MedStar Health Research Institute found that, at an average 9-month follow up in patients enrolled in SOLOIST-WFH, the drug demonstrated cost-effectiveness along with the robust clinical results. Citing commonly accepted willingness-to-pay thresholds in patients with diabetes and worsening heart failure, and in accordance with the Consolidated Health Economic Evaluation Reporting Standards, MedStar’s analysis was designed to extrapolate patients’ life expectancy and life quality to estimate a cost-effectiveness of the drug.
Sources:
- https://www.lexpharma.com/media-center/news/2023-05-26-lexicon-announces-fda-approval-of-inpefa-sotagliflozin-for-treatment-of-heart-failure
- https://www.acc.org/about-acc/press-releases/2021/05/17/03/52/sotagliflozin-shows-benefit-for-difficult-to-treat-form-of-heart-failure