Not only did Cardiometabolic Health Congress (CMHC) chairperson Anne L. Peters, MD, write the book (actually, several) on managing prediabetes and diabetes, she is passionate about staying on the cusp of research – from lifestyle interventions to new therapies – in cardiometabolic medicine. As one of the most influential and well-respected figures in the field, Dr. Peters is relied on by many for her opinions on emerging therapeutics and exciting research developments in diabetes, obesity, and other conditions in the cardiometabolic spectrum.
Meet Dr. Peters
As the director of the Westside Center for Diabetes and professor in the Keck School of Medicine at the University of Southern California in Los Angeles, Anne L. Peters, MD, is one of the world’s leading clinicians and researchers in diabetes care. She works with the L.A. County Department of Health Services on a county-wide diabetes program, and she established the Community Diabetes Initiatives Research Center with Children’s Hospital Los Angeles. For her tireless efforts in improving outcomes for patients with diabetes and related illnesses, Dr. Peters has received the American Diabetes Association (ADA) Outstanding Physician Clinician Award, and for her work with underserved populations, she received the Bernardo Houssay Award from the National Minority Quality Forum.
A sought-after speaker and thought leader, Dr. Peters is instrumental in developing national standards for diabetes care, serving on the Guidelines Committee for Management of Type 1 Diabetes for the ADA and the European Association for the Study of Diabetes (EASD). She also participates in the EASD/ADA Committee on Device Safety and chairs the Endocrine Society Committee on the Use of Devices in Treatment of Diabetes. As CMHC chairperson, Dr. Peters is instrumental in curating each year’s agenda and hand-selecting topics she has identified as the most relevant and up-to-the-minute in her specialty of endocrinology and diabetes. Here, Dr. Peters shares some exciting developments in the fields of type 1 diabetes (T1D) and type 2 diabetes (T2D) that practitioners should be closely following.
The latest in T1D
- Automated insulin delivery. The iLet system is a method of automated insulin delivery that can help people with T1D by delivering the appropriate dose of insulin based on the patient’s weight and food intake at each meal. A recent study comparing the iLet with other automated insulin delivery systems showed promising results, especially on overnight blood glucose levels, which are harder to control. The results of the present study were detailed at the Advanced Technologies & Treatments for Diabetes meeting. In reference to the iLet study Dr. Peters, ever the champion for more diversity in clinical trials, commented, “One of the main reasons I think this study was so cool is because it included over 25% minority individuals who aren’t routinely studied in these insulin device trials.” Dr. Peters noted that the significance of this study might be simply that more automated systems are being studied, being made available, and being improved in terms of usability for both patients and physicians.
- A cure for T1D? Not exactly, but we are getting close. The newest advances in regenerative medicine and biopharma have demonstrated that stem cell-derived differentiated islet cells can be infused into humans and result in new islet cell formation capable of producing insulin. Dr. Peters commented, “Have we cured type 1 diabetes? No, but we are advancing in terms of our treatments for type 1 diabetes, and I think we’re closer than we ever have been to making it much easier for people to live with type 1 diabetes. The development of these new therapeutic approaches is incredibly exciting, and I think in the next few years we’ll learn newer, novel, and better ways for helping our patients with type 1 diabetes.”
The latest in T2D
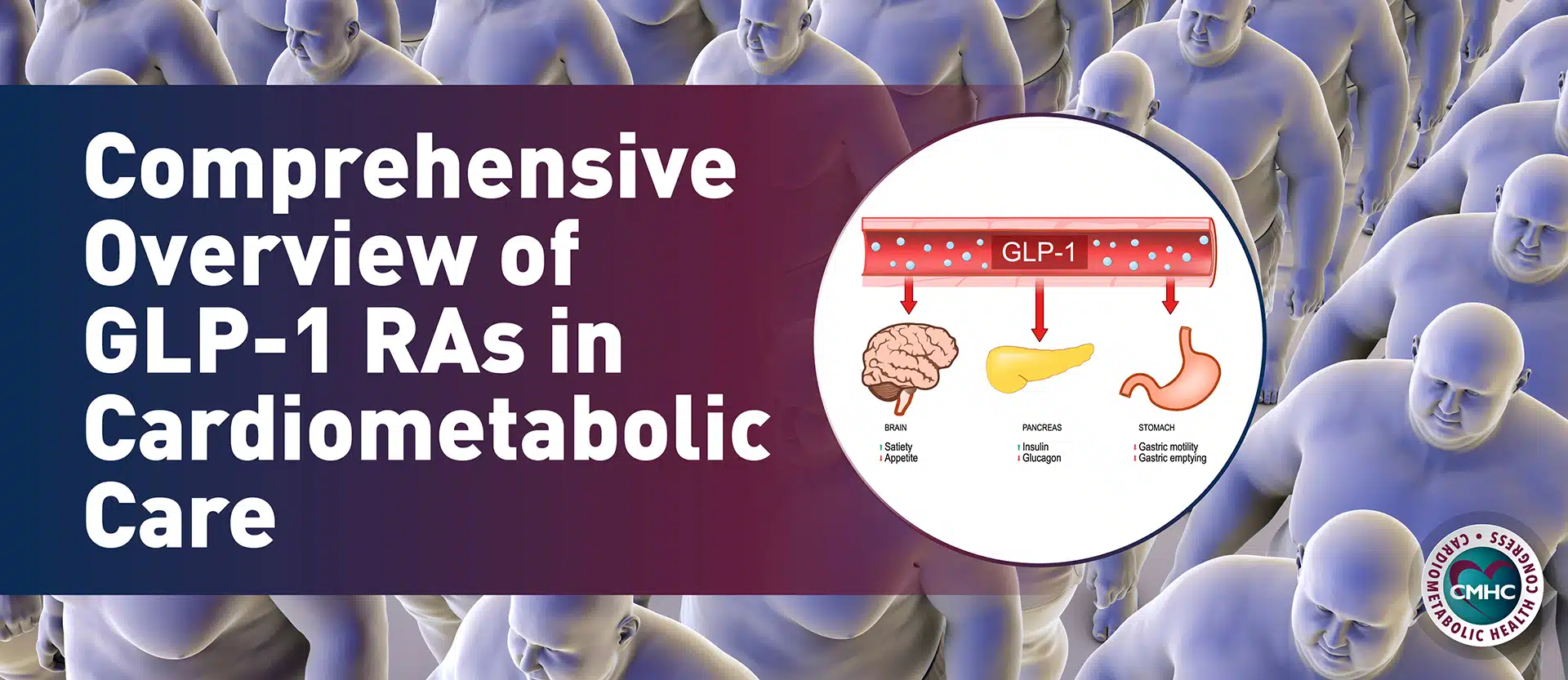
- GLP-1 agonists. There are several glucagon-like peptide-1 (GLP-1) agonists on the market for both T2D and obesity. These include once-weekly injection or once-daily oral semaglutide, once-weekly injection dulaglutide, twice-daily injection exenatide, and daily injection liraglutide. According to Dr. Peters and the ADA, metformin remains the recommended first-line therapy for treating T2D, “however, the addition of a GLP-1 analog should be considered in patients with a contraindication or intolerance to metformin, in patients with a hemoglobin A1c greater than 1.5% over target, or in patients who do not reach their target A1c in three months, particularly in patients with atherosclerosis, heart failure, or chronic kidney disease.”
- Tirzepatide. Just approved by the U.S. Food and Drug Administration (FDA) as the first dual GIP/GLP-1 receptor agonist to treat T2D, tirzepatide’s results in the SURPASS trial showed an average reduction in A1c of 1.6% from baseline, as well as weight. “I’m really excited about this drug. I think it’s going to help a lot of our patients who still struggle with high A1c levels and in particular, those patients who may be on insulin, patients on insulin who have trouble dosing their insulin or adjusting their insulin. Maybe this will be useful in getting them on either simpler insulin regimens or less insulin.”
-
- Weight loss. “We’ve also seen significant weight loss with tirzepatide, which is greater than the agents it was studied against. I think these data are really exciting, and they’re the data that the FDA used to approve the drug,” commented Dr. Peters. According to Lilly, the makers of tirzepatide, more than half of patients on a high-dose injection of the drug lost weight.
- Cardiovascular benefit. Tirzepatide is currently being studied for any potential cardiovascular outcomes, for which Dr. Peters is very interested: “The one caveat to remember is that it does not have the cardiovascular outcomes indication. And when you have patients with type 2 diabetes and known cardiovascular disease, you may want to stay with an agent that does have a cardiovascular benefit, at least until we have the cardiovascular outcomes trial available with tirzepatide.”
- Updated guidelines. As a part of the ADA’s Professional Practice Committee, Dr. Peters is instrumental in updating the Standards of Care in diabetes management. The committee reviews all publications pertaining to diabetes and appoints subcommittees to comb through data to develop their recommendations. The latest updates in 2022 focus on the use of GLP-1 receptor agonists and SGLT2 inhibitor use, and emphasize collaboration between endocrinologists, nephrologists, and cardiologists when managing patients with cardiometabolic comorbidities. There is also an emphasis in the new guidelines for individualizing care: “When it comes to diabetes, we have many good ways to lower glucose levels. I think it’s important to be mindful of the entire patient, their circumstance, and how it is best to treat that individual,” said Dr. Peters.
Key takeaway
Dr. Peters is leading the charge to improve access to care and outcomes for all individuals with diabetes through her research, advocacy, instruction, and influence in the industry. As CMHC chairperson, she shapes the agenda in this important topic area, allowing attendees from all over the world the benefit of her insights and experience. You can see Dr. Peters in person October 19-22, 2022, when presents at the 17th Annual CMHC in Boston.