Introduction
Measurement of glucose level is a cornerstone of effective diabetes management. Glycated hemoglobin (HbA1c) testing, the reference standard for assessment of overall glycemic control and prediction of long-term complication risk, has recognized limitations that include inability to provide information regarding the magnitude and frequency of blood glucose variability. Limitations are also recognized for self-monitoring of blood glucose (SMBG), a central tool in diabetes management, since it provides only a single measurement of glucose that is devoid of context for direction or rate of change.1 Continuous glucose monitoring (CGM) technology addresses limitations of both HbA1c and SMBG by providing near-real time data with information on both glycemic fluctuations and trends, along with assessment of overall blood glucose control.2
CGM sensors have evolved to overcome difficulties with accuracy demonstrated by early devices.2 CGM systems are now an established tool for blood glucose control and management of patients with type 1 diabetes (T1D) and type 2 diabetes (T2D) treated with intensive control regimens.2,3 This clinical brief explores CGM use for patients with T2D, particularly those on less intensive insulin treatment regimens.
Evolution of CGM Systems to Improve Accuracy
Continuing advancement of sensor technology has occurred since the launch of the first CGM system in 1999. To assess the accuracy of CGM sensors, CGM tracing data were compared with reference blood glucose concentration values obtained using laboratory-grade medical instruments such as the Yellow Spring Instruments Inc (YSI).2 The mean absolute relative difference (MARD) value, defined as the average of the absolute error between all CGM values and matched reference values remains the most commonly used metric for this purpose. Lower MARD values are associated with greater device accuracy. Early CGM systems had limitations due to poor accuracy, as systems available around 2011 had MARD values between 13-14.5%, much above the typical MARD of SMBG devices of 5-10%.2
More recent CGM systems have improved upon the lack of accuracy demonstrated by initial devices. For example, the Medtronic Enlite CGM system released in 2011 achieved a MARD of 13.6%. By 2017, The Medtronic Guardian Sensor 3 was released with MARD of 9.1%, within the SMBG accuracy range. The Dexcom G4, G5, G6 systems, released between 2015-2018 each have MARD values of 9-10%.2 The Dexcom G7, cleared by the FDA in December 2022, has a MARD of 8.2%.4
CGM Systems Available in 2023
Current CGM technologies are classified either for professional or personal use. Specific characteristics and benefits can be identified for each category.2,5
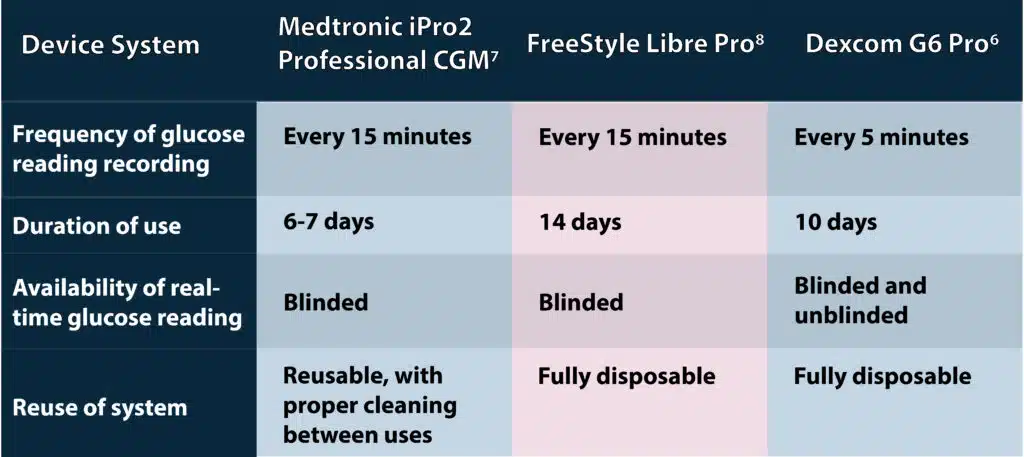
- Professional CGM. Professional CGM (also known as diagnostic CGM) devices are owned by medical systems or practices and are intermittently prescribe to patients as a diagnostic tool.2,5 Collected blood glucose values are not displayed to patients with professional CGM systems (with the exception of an option for display with the Dexcom G6 Pro device6) and alarms for values outside of the target range do not occur.2,5-8 Retrospective analysis of blood glucose data by caregivers allows identification of glucose patterns, correlation with dietary and daily living habits, and assessment of variability metrics, fostering informed adjustment of therapeutic regimens.2, 5
Features of the 3 professional CGM systems approved by the FDA and currently available in the United States are presented in Table 1.
Professional CGM can be helpful in the following specific clinical situations: 2,5
- Unstable blood glucose patterns with excessive hyperglycemia/hypoglycemia
- Discordant results for HbA1c values compared with SMBG values*
- Unexplained fasting hyperglycemia
- Need to verify the effect of changes to the diabetes care plan
- Assessment of dietary modification
- Lack of adequate SMBG data
- Patient concerns regarding readiness for personal CGM
- Lack of insurance coverage for personal CGM
Benefits of professional CGM use were recently demonstrated in a real-world quality improvement project carried out from 2017-2018 in a primary care setting with a physician or RN/certified diabetes educator caregiver. For 68 participants with T2D on any treatment regimen, use of professional CGM was associated with a reduction of HbA1c from 8.8% ± 1.2% to 8.2% ± 1.3% (p=0.006). In addition, in this care model, time in range and reduction of time in hyperglycemia were demonstrated without necessarily requiring additional medications.10
*Regarding the accuracy of HbA1c measurements, a substantial discordance was demonstrated in a recent comparison of laboratory measured HbA1c versus estimated HbA1c calculated using the glucose management indicator feature available on continuous glucose monitoring systems. In a retrospective, real-world study of 641 office encounters from 2012 to 2019, differences between the measurements of HbA1c were ³0.5% in 50%, ³1% in 22%, and <0.1% in 11%, indicating that HbA1c is not a perfect biomarker and may not reflect mean glucose values as accurately as previously postulated.9
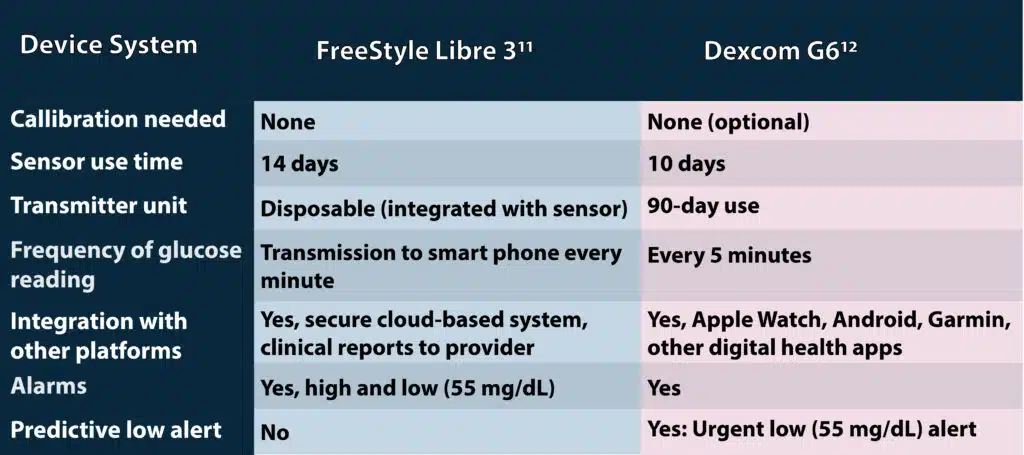
- Personal CGM. Among the 7 personal CGM systems currently available in the United States, the FreeStyle Libre 3 and the Dexcom G6 are approved for non-adjunctive use, indicating that treatment decisions can be made using CGM readings without confirmation by SMBG data.2, 11, 12 The Dexcom G7, not yet available to the consumer but anticipated in 2023, will have nonadjunctive use similar to the G6.4 Table 2 summarizes key features of the non-adjunctive use personal CGM systems.
Personal CGM systems may also be categorized as real-time (rt) CGM or intermittently scanned (is; also known as flash) CGM. Rt-CGM devices transmit sensor data continuously to a display device, and alerts and alarms occur without user intervention, while is-CGM requires the user to scan or swipe the sensor with a reading device in order to initiate transfer of data from the sensor to the display device and no automatic alerts or alarms occur.13
Current Guideline Recommendations for CGM
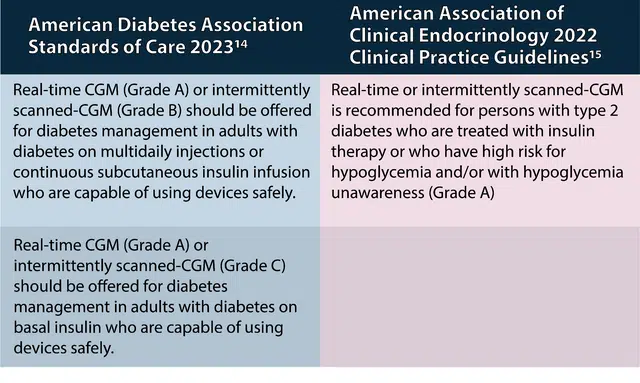
Both the American Diabetes Association (ADA) and the American Association of Clinical Endocrinology (AACE) include recommendations for use of CGM in patients with T2D in current guidelines, as summarized in Table 3.14, 15
Key Concepts for Successful CGM
Real-time data provided by CGM ideally can inform diabetes treatment decisions, reveal trends with advanced warning of rapid shifts, and provide motivation for enhanced diabetes self-management.16 However, to derive maximal benefit from the information provided by CGM, training of patients and caregivers is imperative because effective use requires the user to interpret the collected data and respond accordingly with changes.13
Key patient education topics for optimal CGM use include: 13, 16,17
- Proper sensor insertion technique, maintenance, and trouble-shooting
- Calibration techniques and timing (if needed)
- Recognition of CGM data patterns and trends, so that blood sugar changes can be correlated with specific causes
- Appropriate settings for alerts, alarms, and thresholds
- Data transmission and sharing
- Use of predictive alerts and trend-based insulin dose adjustments.
Patients may become overwhelmed by the amount of information generated by CGM and become desensitized to the information and notifications provided, with potential development of alarm fatigue. To alleviate this, it may be helpful to set device alarms in stages, with hypoglycemia alerts as the first priority and postponement of hyperglycemia alert establishment. Setting alarms based on the individual needs of each patient is another strategy to diminish alarm fatigue; for example, beginning with higher settings for patients with blood glucoses routinely above the target range, with subsequent adjustments to lower settings as control improves.16
Patients should be aware that when the blood glucose concentration is rapidly changing, a lag between interstitial glucose values (upon which CGM values are based) and blood glucose values may occur in certain circumstances, including postprandially and following exercise or correction of hypoglycemia. Studies in CGM users with T1D have demonstrated that detection of blood glucose decline during prolonged aerobic exercise lags behind detection of decline by SMBG, prompting the recommendation to confirm glucose levels by SMBG when hypoglycemia is suspected during exercise.18
Since multiple CGM systems are available, it is important that patients receive education that is focused on the details of their specific system from caregivers that are appropriately familiar with that device.13,16 Conventions for displaying information on CGM devices are not yet standardized. For example, device arrows that convey rate of change information vary in meaning among devices and universal methods to adjust insulin dose based on trend arrows are not available, underscoring the need for education that is specifically matched to the individual patient and device.19,20
Core CGM Metrics
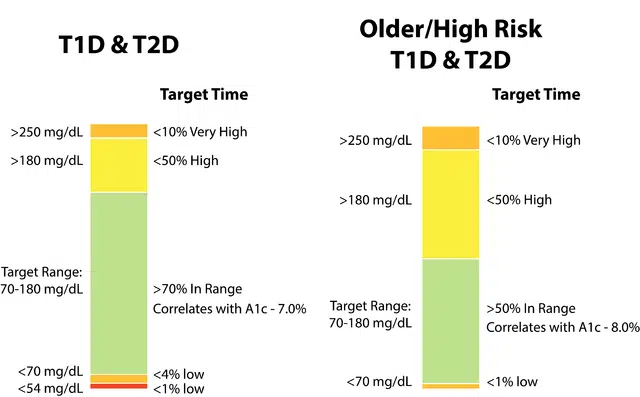
Commitment to establish and achieve blood sugar target goals that are agreed upon by patient and caregivers gives meaning to collected CGM data. Time in range has been deemed by international consensus as a metric of glycemic control that provides more actionable information than HbA1c alone. Three key CGM measurements comprise the time in range metric:
- Time in range (TIR): percentage of reading and time per day within the target glucose range
- Time below target range (TBR)
- Time above target range (TAR)
Expectations for the various time in range measures are established based on safety concerns and goals of the current treatment plan. In general, the first priority is to minimize hypoglycemia, resulting in reduction of TBR and more time in the target range; efforts to reduce TAR and maximize TIR typically follow.21 Figure 1 illustrates how goals for core CGM metrics may be adjusted based on characteristics such as age and risk status for patients with diabetes.
Additional Key Metrics That Characterize CGM Use
- Number of days with CGM data and percentage of time using CGM is active another metric to characterize CGM use. Greater than 70% use over the most recent 14 days is recommended, as this has been demonstrated to correlate strongly with 3 months of mean glucose measurement.
- Glucose management indicator (GMI) provides a calculated HbA1c level based on average glucose measured by CGM parameters
- Coefficient of variation (CV) is a measure of glycemic variability; £ 36% is recommended.
Evidence for Benefit of CGM for T2D Treated with Non-intensive Insulin
- Clinical Trial Data. While clinical benefit for CGM is well established for people receiving intensive insulin therapy for T1D or T2D, the benefit of CGM in T2D with non-intensive insulin treatment is less clear. However, recent evidence supports benefits of CGM across a spectrum of insulin treatment regimens. The DIaMonD T2DM Study (NCT02282397) provides evidence for benefit of CGM for patients with T2D treated with multiple daily injections of insulin. DIaMonD T2DM was a 24-week randomized controlled trial of 158 adults with T2D treated with multiple daily injections of insulin comparing CGM with usual care. Participants had HbA1c levels of 7.5% to 9.9% (mean, 8.5%) and the primary endpoint was HbA1c reduction at 24 weeks. At 24 Weeks, mean HbA1c levels decreased to 7.7% in the CGM group vs 8.0% in the control group (P = .022) with no difference between the groups in rate of hypoglycemia or quality-of-life measures. The authors concluded that CGM can be beneficial for adults with T2D treated with multiple daily injections of insulin.22
Improvement in HbA1c levels with CGM use have also been demonstrated in adults with poorly controlled T2D treated in the primary care setting with basal insulin only. The MOBILE study (NCT03566693) was a randomized multicenter clinical trial of 175 racially and socioeconomically diverse participants treated in the primary care setting with basal insulin only, randomized in a 2:1 ratio to CGM (n=116) or traditional SMBG (n=59). The primary outcome was HBA1c level at 8 months. CGM, as compared with SMBG, resulted in significantly lower HbA1c levels. HbA1c decreased from 9.1% at baseline to 8.0% at 8 months in the CGM group, compared with a decrease from 9.0% to 8.4% in the SMBG group (adjusted difference, -0.4% [95%CI, -0.8% to -0.1%]; P = .02). No difference in the rate of severe hypoglycemic events was noted between the groups. Mean percentage of TIR 70-180 mg/dl at 8 months was 59% for the CGM group versus 43% for the SMBG group (adjusted difference, 15% [95% CI, 8% to 23%]; P < 0.001), which equates to 3.6 hours more per day with TIR 70-180 mg/dl for the CGM group.23
An extension of the MOBILE study explored the effect of discontinuing CGM after 8 months of CGM use in adults with T2D treated with basal insulin only. After completion of the MOBILE study, the CGM group was randomly reassigned either to continue CGM (n=53) or discontinue CGM with resumption of SMBG (n=53) for an additional 6 months of study. The original SMBG group continued with SMBG for this continuation study. For those who had been using CGM, discontinuation of CGM resulted in a 12% loss of TIR compared with a 1% gain in TIR for the group that continued CGM. The authors summarize that the MOBILE extension study demonstrated the benefit of CGM for patients with T2D treated with basal insulin only through 14 months.24
- Real-World Evidence (RWE) Supporting Benefit for CGM. RWE obtained from studies that analyze data mined from a variety of sources related to patient health status or the delivery of health care, such as electronic health records, claims and billing activities, and disease registries, are having an increasing role in regulatory decisions and development of guidelines and treatment approaches.25
RWE has been generated supporting benefits for use of CGM for patients with T2D. A retrospective cohort study of commercial and Medicare supplemental database claims throughout the US in 2463 individuals with T2D treated with insulin therapy demonstrated that acute diabetes-related events and all-cause inpatient hospitalizations significantly decreased during the first 6 months after acquiring flash CGM (FreeStyle Libre), compared with event rates during the 6 months prior to acquisition of CGM, providing support for the use of flash CGM to improve outcomes for T2D patients on insulin. 26 Similar benefit was demonstrated in the RELIEF study, a large retrospective analysis of 74,011 flash CGM users with T1D or T2D identified in the French national claims database. Results of the RELIEF study demonstrated that assessed rates of hospitalization for diabetic ketoacidosis and diabetes-related coma were significantly lower compared with the incidence prior to initiation of flash CGM.26
The European diabetes prospective follow-up (DPV) registry was analyzed to identify 1440 people with T2D (46% receiving insulin) that initiated CGM in 2015 or later. Comparison of HbA1c levels and body mass index after 3 and 6 months of CGM use with rates in the year before CGM initiation generated RWE that CGM initiation was associated with significant decrease in HbA1c with no change in BMI for patients with T2D. The authors suggest that based on these data, strengthening of patient and physician readiness toward CGM use in T2D should follow.28
Future Directions for CGM Development: Pursuit of Noninvasive Glucose Monitors
Emerging technologies to continuously measure blood glucose in a noninvasive manner are harnessing wavelengths in the electromagnetic spectrum that are beyond the visible light range of 400-700 nm. The Israeli company Cnoga Medical is developing a personalized noninvasive blood glucose measuring device that is based on four light-emitting diodes (LEDs) with discrete wavelength from 600-1000 nm (ultraviolet light range) projected through the fingertip. Calibration for at least the initial 3-day period with standard SMBG is required and the recently reported MARD for the device is 14-17%. Certified and commercialized in Europe, Asia, and South America, this device is in the FDA submission process in the US.29
Know Labs of Seattle, Washington is developing non-invasive device to measure glucose levels through the skin using electromagnetic energy in the form of radio waves. This device is currently undergoing scientific research validation prior to FDA clinical trials.30 The Gwave device, developed by the Israeli company Hagar, also uses electromagnetic radio frequency waves to measure blood glucose levels continuously and in real-time. The device received a breakthrough device designation from the FDA in late 2021 and further clinical trials are planned.31
Conclusion
CGM provides benefit for patients with T2D treated with either intensive or less intensive insulin therapy, as established by data from clinical trials and real-world evidence. While both professional and personal CGM have roles in the management of T2D, personalized education and training with ongoing patient support are critical for optimal use of either approach. Emerging noninvasive technology for real-time CGM are promising.
- Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care.2017;30:1631-1640.
- Cappon G, Vettoretti M, Sparacino G, Facchinetti A. Continuous glucose monitoring sensors for diabetes management: A review of technologies and applications. Diabetes Metab J. 2019;43:383-397.
- Arguello V, Freeby M. Continuous glucose monitoring in insulin injections: Does this mean continuous glucose monitoring for everyone? Ann Intern Med. 2017;167(6):436-437.
- Dexcom G7 receives FDA clearance: the most accurate continuous glucose monitoring system cleared in the US. December 8, 2022. Accessed December 19, 2022. https://investors.dexcom.com/news/news-details/2022/Dexcom-G7-Receives-FDA-Clearance-The-Most-Accurate-Continuous-Glucose-Monitoring-System-Cleared-in-the-U.S/default.aspx?_gl=1*bp1ebe*_ga*MTU4MDQ1MDIxNC4xNjcxMzI1OTE0*_ga_S6J885VKT2*MTY3MTMzODU5OS4xLjAuMTY3MTMzODU5OS4wLjAuMA..*_ga_KQDZFFW8BJ*MTY3MTMzODU5OS4xLjAuMTY3MTMzODU5OS4wLjAuMA..&_ga=2.154732283.218141417.1671325914-1580450214.1671325914
- Vigersky R, Shrivastav M. Role of continuous glucose monitoring for type 2 in diabetes management and research.J Diabetes Complications. 2017;31(1):280-287.
- Dexcom. G6 Pro CGM System. Accessed December 17, 2022. https://provider.dexcom.com/products/dexcom-g6-pro#key-features
- Medtronic. iPro®2 Professional Evaluation CGM System. Accessed December 17, 2022. https://www.medtronic.com/ca-en/diabetes/home/products/cgm-systems/ipro2-professional.html
- Abbott. FreeStyle Libre Pro. 2022. Accessed December 17, 2022. https://www.freestyle.abbott/in-en/libre-pro.html?gclid=COGkh5qMgPwCFU2kHwods08DpA
- Perlman JE, Gooley TA, McNulty B, Meyers J, Hirsch IB. HbA1c and glucose management indicator discordance: a real-world analysis. Diabetes Tech Therap. 2021;23:253-258.
- Simonson GD, Bergenstal RM, Johnson ML, Davidson JL, Martens TW. Effect of professional CGM (pCGM) on glucose management in type 2 diabetes patients in primary care.J Diabetes Sci Technol. 2021;15(3):539-545.
- FreeStyle Libre 3. Introducing the new FreeStyle Libre 3 system. May 2022. Accessed December 19, 2022. https://www.freestyleprovider.abbott/us-en/freestyle-libre-3.html
- Dexcom G6 CGM System for Personal Use. 2022. Accessed 12.19.2022. https://provider.dexcom.com/products/dexcom-g6-personal-cgm-system
- Petrie JR, Peters AL, Bergenstal RM, et al. Improveing the clinical value and utility of CGM Systems: Issues and recommendations. Diabetes Care.2017;40:1614-1621.
- ElSayed NA, Aleppo G, Aroda VR, et al. American Diabetes Association. 7. Diabetes technology: Standards of medical care in diabetes-2023. Diabetes Care. 2023;4a6(Suppl.1):S111-127.
- Blonde L, Umpierrez GE, Reddy SS, et al. American Association of Clinical Endocrinology Clinical Practice Guideline: Developing a diabetes mellitus comprehensive care plan-2022 Update.Endocr Pract. 2022;28(10):923-1049.
- Association of Diabetes Care and Education Specialists (ADCES). The diabetes care and education specialist’s role in continuous glucose monitoring. March 2021. Accessed December 19, 2021. https://www.diabeteseducator.org/docs/default-source/practice/practice-documents/practice-papers/diabetes-care-education-role-in-continuous-glucose-monitoring.pdf?sfvrsn=448e8458_10
- Aleppo G, Webb K. Continuous glucose monitoring integration in clinical practice: A stepped guide to data review and interpretation.J Diabetes Sci Technol. 2019;13(4):664-673.
- Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes.Diabetes Technol Ther. 2019;21(6):313-321.
- Kudva YC, Ahmann AJ, Bergenstal RM, et al. Approach to using trend arrows in the FreeStyle Libre Flash glucose monitoring systems in adults.J Endocr Soc. 2018;2(12):1320-1337.
- Aleppo G, Laffel LM, Ahmann AJ, et al. A practical approach to using trend arrows on the Dexcom G5 CGM system for the management of adults with diabetes.J Endocr Soc. 2017;1(12):1445-1460.
- Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593-1603.
- Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial.Ann Intern Med. 2017;167(6):365-374.
- Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial.JAMA. 2021;325(22):2262-2272.
- Aleppo G, Beck RW, Bailey R, et al. The effect of discontinuing continuous glucose monitoring in adults with type 2 diabetes treated with basal insulin.Diabetes Care. 2021;44(12):2729-2737.
- US Food and Drug Administration. Real-world data (RWD) and real-world evidence (RWE) are playing an increasing role in health care decisions. December 12, 2022. Accessed January 4, 2023. https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence
- Bergenstal RM, Kerr MSD, Roberts GJ, Souto D, Nabutovsky Y, Hirsch IB. Flash CGM Is associated with reduced diabetes events and hospitalizations in insulin-treated type 2 diabetes.J Endocr Soc. 2021;5(4):bvab013. doi:10.1210/jendso/bvab013
- Roussel R, Riveline JP, Vicaut E, et al. Important drop in rate of acute diabetes complications in people with type 1 or type 2 diabetes after initiation of flash glucose monitoring in France: the RELIEF study.Diabetes Care. 2021;44(6):1368-1376.
- Lanzinger S, Best F, Bergmann T, et al. Dynamics of hemoglobin A1c, body mass index, and rates of severe hypoglycemia in 4434 adults with type 1 or type 2 diabetes after initiation of continuous glucose monitoring.Diabetes Technol Ther. 2022;24(10):763-769.
- Cnoga Digital Care. CoG-hybrid glucometer. Accessed January 4, 2023. https://www.cnogacare.co/cog-hybrid-glucometer
- Know Labs. Bio-RFID. Accessed January 4, 2023. https://www.knowlabs.co/bio-rfid
- HAGAR Tech. GWave Glucose. Accessed January 4, 2023. https://www.hagartech.com