A peer-reviewed study circulated by the Journal of the American Medical Association on Monday, May 22, 2023, took a closer look at study results first released in Sept., testing Pfizer’s experimental drug danuglipron. The study enrolled 411 adult participants with type 2 diabetes. Over 16 weeks, patients who took a high dose of Pfizer’s new drug lost an average of 10 pounds.
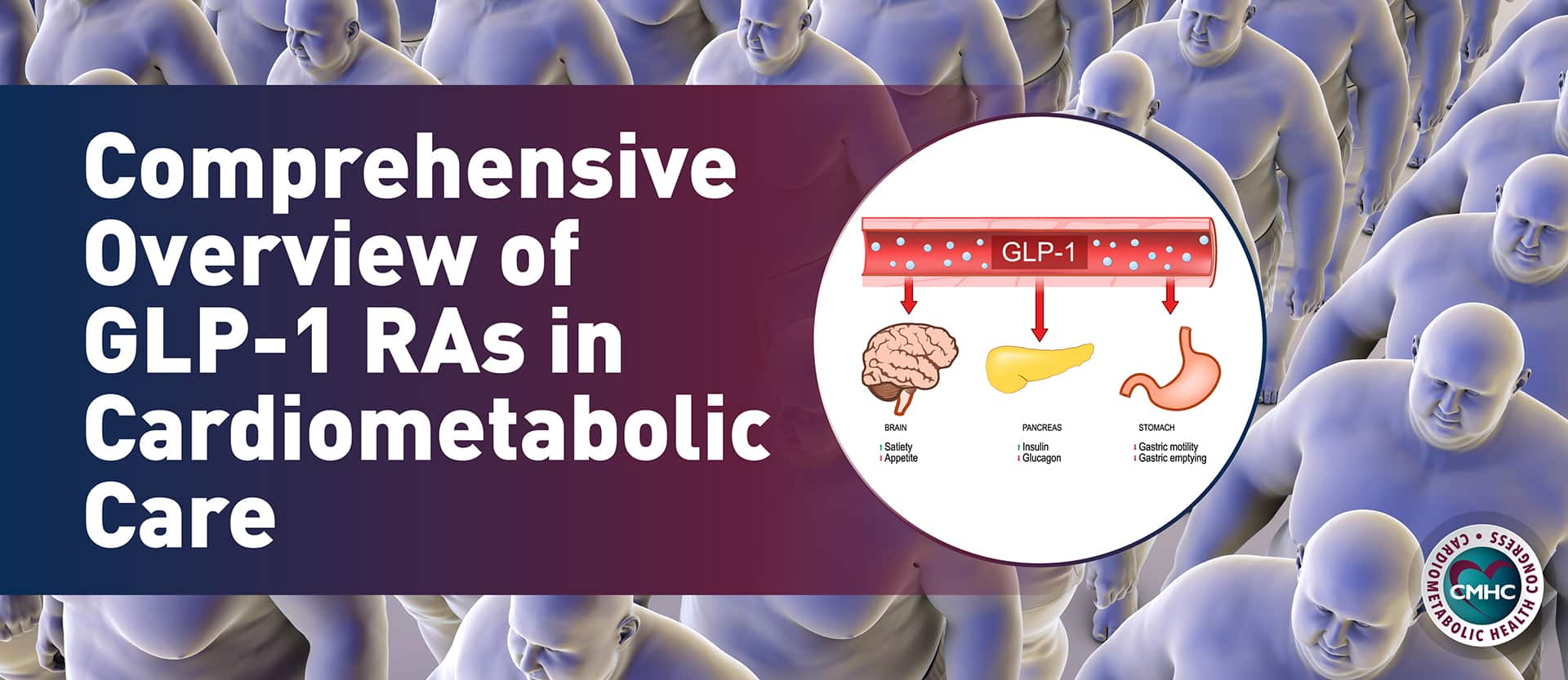
Both Pfizer’s oral drug danuglipron and Novo Nordisks’s semaglutide (Ozempic brand for diabetes, Wegovy brand for weight loss) mimic glucagon-like peptide-1 (GLP-1), a gut hormone that signals the brain when a person is full. It also has an impact on blood sugar, helping the pancreas release insulin.
The trial results
To compare, recipients of high-dose Ozempic lost about the same amount of weight as the danuglipron study group over 30 weeks, according to Novo Nordisk’s Phase 3 study of the semaglutide injectable formulation.
Over the course of just 16 weeks, danuglipron users experienced the following metabolic benefits:
- An average reduction in A1C of 1.16%
- An average fasting plasma glucose reduction of 33 mg/dL
- An average weight loss 4.17 kgs (9.2 pounds)
The first pivotal trial of injectable semaglutide (Ozempic) delivered similarly impressive results, but in a much longer 30-week trial period:
- An average reduction in A1C of 1.55%
- An average fasting plasma glucose reduction of 20 mg/dL
- An average weight loss of 4.53 kgs (9.99 pounds) in a similar population
Is there an oral form of semaglutide?
Yes, an oral form of semaglutide is available as a daily pill under the name Rybelsus. For comparison, the six-month trial of the highest dose of Rybelsus helped people with type 2 diabetes achieve:
- An average reduction in A1C of 1.3%
- An average weight loss of about 10 pounds after one year
These numbers are slightly more impressive than the danuglipron results, but there is a possibility that danuglipron users would have reached the same weight loss and A1C improvement had they been followed for the 6 to 12 months Rybelsus users were.
Although it seems as powerful as injectable Ozempic and oral danuglipron ounce for ounce, it has a notable drawback. Rybelsus is something of a hassle to take — it must be swallowed on an empty stomach exactly 30 minutes before eating or drinking anything other than water, and before using any other oral medications. Because danuglipron can be taken with or without food it may be more user-friendly and thereby have better adherence and outcomes in real-life settings.
Side effects of GLP-1s
Most GLP-1 receptor agonists, including semaglutide, have associated gastrointestinal effects. According to Pfizer, nearly half of danuglipron users reported at least one gastrointestinal side effect, and 34% discontinued the medication because of a treatment-emergent adverse event. The following side effects were the most commonly reported among participants taking the highest danuglipron dosage:
- 32% of users reported nausea
- 25% of users reported vomiting
- 10% of users reported diarrhea
Similarly, when taking the maximum-strength dosage of oral semaglutide available, patients also reported high rates of gastrointestinal side effects. According to a Rybelsus trial published in the The New England Journal of Medicine:
- 44% of users reported nausea
- 32% of users reported diarrhea
Key takeaway
Early evidence showed that Pfizer’s new oral GLP-1 receptor agonist danuglipron showed similar results to Novo’s Ozempic without the need for injection, and to its oral formulation Rybelsus without the need for fasting restrictions. The high prevalence of gastrointestinal adverse effects with any of these agents must be weighed against the benefits of weight loss and the associated improvement in cardiometabolic comorbidities; the effective use of GLP-1s will rely heavily on the shared decision-making between providers and patients.
Sources:
- Wilding, John P.H. Once-Weekly Semaglutide in Adults with Overweight or Obesity. New England Journal of Medicine. March 18, 2021 384(11):989. doi 10.1056/NEJMoa2032183 / https://www.nejm.org/doi/full/10.1056/NEJMoa2032183
- Saxena AR, Frias JP, Brown LS, et al. Efficacy and Safety of Oral Small Molecule Glucagon-Like Peptide 1 Receptor Agonist Danuglipron for Glycemic Control Among Patients With Type 2 Diabetes: A Randomized Clinical Trial. JAMA Network Open. 2023;6(5):e2314493. doi:10.1001/jamanetworkopen.2023.14493