Experts in obesity medicine overwhelmingly agree that advising patients to “eat less, move more,” doesn’t result in meaningful weight loss. The growing number of adults and children with obesity indicates that providers must rely more heavily on the landscape of newly approved pharmaceutical agents to control the worldwide obesity crisis. And, the most promising agents for weight loss appear to be diabetes drugs that were initially developed to improve glycemic control but were found to help patients lose weight, too.
According to the American Diabetes Association (ADA), more than 37 million people in the US have diabetes, and more than 100 million have obesity. Nearly 90% of patients in the US with diabetes also have overweight or obesity, and an estimated half of all people in the US will have obesity by 2030. Providers who see patients with obesity, type 2 diabetes (T2D), and related risk factors such as hypertension, hyperlipidemia, cardiovascular disease, chronic kidney disease (CKD) and more, know that these conditions exist in a complex constellation of cause-and-effect organ damage.
"We learn energy balance. We learn about calories in (our food and beverage intake) and calories out (our bodily secretions and our physical activity). And if we can just get this right, eat the right amount, exercise the right amount, then we should be the weight we want to be, right? This is wrong. It’s so much more complex. There are things that are influencing or inhibiting your energy intake, things like leptin and other dipeptides and appetite-inhibiting hormones, and then there are things that increase our demand to eat, like ghrelin, an appetite-stimulating hormone. All of these interact with our GI tract, our exercise and our energy balance, and regulate how much we weigh."
- Fatima Cody Stanford, MD, MPH,
Lifestyle modifications such as improved diet and increased physical activity, despite being first-line obesity interventions, usually only result in a 5-10% reduction of body weight. A more significant weight loss of 10-25% is seen with bariatric surgery, but at the population level patients may be apprehensive about potential complications or in such poor health they are not eligible for anesthesia. New pharmaceutical agents may fill this treatment gap, resulting in more meaningful weight loss than lifestyle changes alone without the need for invasive surgical interventions.
Glucose control for weight loss
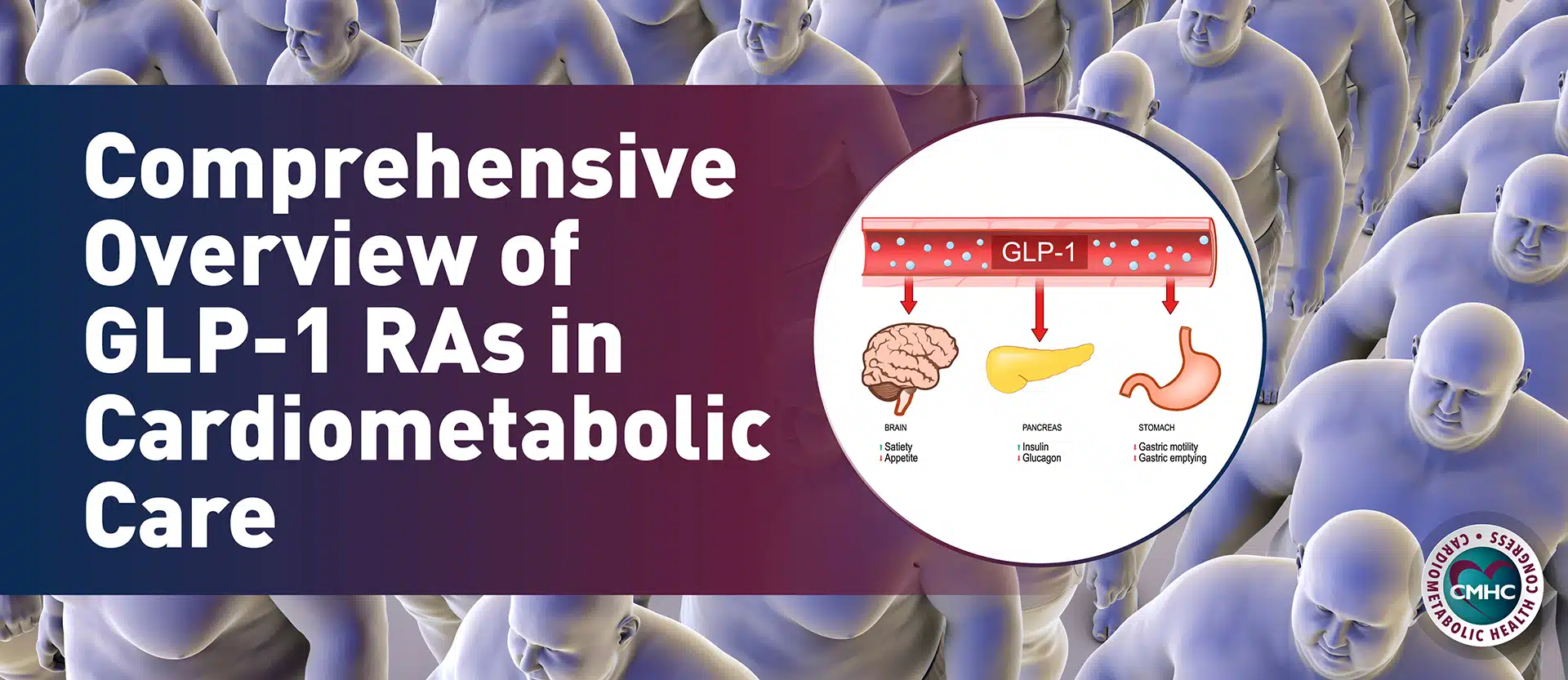
In the search for a drug to control blood glucose in patients with T2D, investigators targeted gut hormones that regulate appetite and glucose homeostasis. They discovered glucagon-like peptide 1 (GLP1), a hormone secreted mainly from the ileum that reduces appetite, delays gastric emptying, increases insulin secretion and inhibits glucagon secretion.
Because patients taking GLP1 receptor agonists experienced better glucose control and significant weight loss, higher doses for the treatment of obesity have been studied in recent years with significant short-term success. The potential for long-term weight loss maintenance is now one of the biggest areas of interest in the use of GLP1s to treat obesity.
Liraglutide
Developed under the brand name Victoza, GLP1 receptor agonist liraglutide was initially approved in the US as a second-line therapy (after metformin) for glucose control in T2D. The agent was subsequently approved for weight management under the brand name Saxenda in 2014. Liraglutide is typically administered as a once-daily subcutaneous self-injection and prescribed to individuals with a body mass index (BMI) of 30 or higher or to those with a BMI of 27 or higher who have weight-related health conditions, such as high blood pressure or type 2 diabetes.
Tirzepatide
Already FDA-approved to treat T2D under the brand name Mounjaro, tirzepatide is expected to be approved for weight loss in 2023 in both the US and Europe, after results from the drug’s phase 3 SURMOUNT-2 study revealed the drug achieved an average 15% weight loss in adults, versus 3.2% achieved by patients taking a placebo.
Tirzepatide works by mimicking two naturally produced hormones in the gut called GLP-1 and GIP. The hormones signal to the brain when a person is full, suppressing their appetite. Semaglutide (Ozempic and Wegovy) only target GLP-1, but Eli Lilly plans to pit tirzepatide against Wegovy in 700 patients who have obesity or are overweight with weight-related health conditions. The company expects to complete the study in 2025. Despite the side effects, the FDA’s approval of tirzepatide is a significant development in the fight against obesity. Tirzepatide is a once-weekly injection that works by mimicking the action of a gut hormone called GLP-1, which helps to suppress appetite and control blood sugar levels.
Tirzepatide would be the third GLP-1 receptor agonist to be approved by the FDA for weight loss, following Novo Nordisk’s semaglutide formulation Wegovy.
Semaglutide
Semaglutide, already approved as a once-weekly injectable for weight loss under the brand name Wegovy, was first introduced as a T2D therapy under the now-eponymous Ozempic. Earlier this year, semaglutide was even approved to treat obesity in pediatric patients.
For the first time, a 50-mg oral formulation of semaglutide is being investigated to treat obesity. Researchers found that this once-daily oral semaglutide 50 mg resulted in a superior and clinically meaningful weight loss of 15.1% compared with placebo when used alongside diet and physical activity in adults with overweight or obesity without T2D.
“Having an oral formulation of semaglutide in addition to the subcutaneous, or injectable, formula available will allow people who struggle to lose weight with diet and physical activity alone to take this effective medication in a way that best suits them. The weight loss also led to improvements in physical functioning, allowing participants to have an improved quality of life for everyday activities.”
- Professor Filip K. Knop MD, PhD, University of Copenhagen
Survodutide
Results from a phase 2 trial of survodutide were presented at the 83rd ADA Scientific Sessions, which took place in San Diego in June 2023. Individuals with overweight or obesity without diabetes were randomized to receive the new drug developed by Boehringer Ingelheim and Zealand Pharma showed more significant weight loss than those receiving the placebo. Survodutide is a dual agent that activates both the glucagon and GLP-1 receptors, as tirzepatide does. Patients using survodutide exhibited almost a 18.7% weight loss at 46 weeks, which rivals the results of semaglutide, which demonstrated a 15% weight loss in a similar time frame, but with additional improvement in type 2 diabetes markers, which survodutide did not include as outcomes.
“Given the prevalence of obesity and its many disease-related complications, there is a dire need for treatments that can help treat the disease of obesity effectively. Current treatments for obesity mainly focus on weight loss by reducing energy intake. By activating both the glucagon and GLP-1 receptors, survodutide may both inhibit appetite and improve energy expenditure, thereby helping to treat the disease of obesity. These encouraging data support the further study of survodutide in larger Phase III trials.”
- Carel le Roux, MD, PhD, principal investigator
Key Agents in the Pipeline
Several drugs are in various stages of development and have shown promise in clinical trials. If approved, they could offer even more options for providers looking for effective ways to help their patients lose weight.
- Retratutide. This investigational GLP-1 receptor agonist that seems to offer significant advantages over other existing drugs for weight loss and diabetes management. It may provide greater weight loss, but potential users will need to consider the various health implications the drug may present. Being developed under the brand name Mounjaro LT, retatrutide has a long way to go before it’s available to the public. “It is early days,” Skovronsky said. The drug performed well in phase 2 clinical trials, with participants losing an average of 24% of their body weight. These results were shared by Eli Lilly at the American Diabetes Association’s annual meeting in June 2023. The company also published the study in the New England Journal of Medicine.
The company has begun enrolling participants in a larger phase 3 trial expected to be completed in December 2025. - Semaglutide and cagrilintide. A recent phase 2 clinical trial found a once-weekly injection of the combination drug CagriSema (a compound of semaglutide and the amylin-mimicking cagrilinitide) resulted in an A1C reduction of 2.2%, weight loss of 16%, and time-in-range blood glucose improvements of 43%.
- Pramlintide acetate. A synthetic analog of human amylin, being developed under the brand name Symlin, pramlintide acetate is a naturally occurring hormone made in pancreatic beta cells being investigated for its weight-loss properties. Originally developed to treat type 1 diabetes and first approved for its use in 2005, this agent mimics the hormone amylin which plays an important role in glucose regulation by slowing digestion. Because slower digestion often results in less hunger, the drug is currently being investigated for its use to treat obesity and overweight. Like insulin, which is co-secreted with amylin by beta cells around mealtimes, Symlin may fall also eventually fall into the category of T2D treatment because it minimizes glucose spikes seen in both types of diabetes after meals.
Key Takeaway
The growing number of patients with obesity and overweight indicates that dietary, lifestyle, and physical activity interventions aren’t doing enough to combat genetic and environmental predispositions for weight gain. Introducing new effective weight-loss agents should be considered for patients with obesity, especially when cardiometabolic comorbidities such as T2D, CKD, heart failure, hypertension, or hyperlipidemia are present. Patients are likely to have better medication adherence and weight-loss outcomes when they feel involved in the decision and know that these drugs can be added to their current efforts to improve their diet and lifestyle. While these drugs have all shown impressive results in clinical trials, with some patients losing up to 20% of their body weight, they also have side effects including nausea, vomiting, diarrhea, and constipation.
- Klein, H. E. (2023, July 12). Patients with overweight or obesity show significant weight loss in phase 2 survodutide trial. AJMC. https://www.ajmc.com/view/patients-with-overweight-or-obesity-show-significant-weight-loss-in-phase-2-survodutide-trial
- Pfizer provides update on GLP-1-RA clinical development program for adults with obesity and Type 2 diabetes mellitus. (n.d.). Pfizer.com. Retrieved July 12, 2023, from https://www.pfizer.com/news/press-release/press-release-detail/pfizer-provides-update-glp-1-ra-clinical-development
- Late breaking weight loss innovations: New drug therapies shown to offer positive outcomes for obesity and type 2 diabetes management. (n.d.). Diabetes.org. Retrieved July 12, 2023, from https://diabetes.org/newsroom/press-releases/2023/late-breaking-weight-loss-innovations-new-drug-therapies-shown-offer-positive-outcomes-obesity-type-2-diabetes-management
- EU investigates Ozempic, weight-loss drug Saxenda after suicidal thoughts reported. (2023, July 10). Reuters. https://www.reuters.com/business/healthcare-pharmaceuticals/eu-probes-novos-weight-loss-drugs-reports-suicide-risks-bloomberg-news-2023-07-10/
- Amylin reports weight-loss success with Symlin. (2005, June 3). FierceBiotech. https://www.fiercebiotech.com/biotech/amylin-reports-weight-loss-success-symlin
- Symlin, an injectable form of Amylin. (2010, November 18). Diabetesnet.com; Diabetesnet.com – The Diabetes Mall. https://www.diabetesnet.com/about-diabetes/diabetes-medications/symlin/
Melson, E., Miras, A. D., & Papamargaritis, D. (2023). Future therapies for obesity. Clinical Medicine (London, England), 23(4), 337–346. https://doi.org/10.7861/clinmed.2023-0144