Another drug to help patients with obesity-related conditions lose weight has been approved by the U.S. Food and Drug Administration (FDA). The approval of Zepbound comes at a pivotal time for the drug market, when ballooning interest and short supply have clinicians scrambling to secure (and patients struggling to afford) the new drugs many experts are calling the holy grail of obesity treatment.
The FDA has approved Eli Lilly’s new tirzepatide formulation (Zepbound) for chronic weight management in adults. On Nov. 8, 2023, the company announced that Zepbound had gotten the green light for use in patients with obesity (body mass index >30) or overweight (body mass index > 27) with at least one other weight-related cardiometabolic condition such as high blood pressure, type 2 diabetes or high cholesterol. The FDA noted that it recommends the use of agents such as Zepbound in addition to a reduced-calorie diet and increased physical activity because just a 5-10% reduction in body weight is associated with a reduced risk of cardiovascular disease in adults with obesity or overweight, who now make up approximately 70% of the U.S. population.
Background
Tirzepatide, the active ingredient in Zepbound, is also the active ingredient in Lilly’s already-approved Mounjaro, a drug to improve blood glucose control in adults with type 2 diabetes. Before Zepbound’s approval, many providers had already been prescribing Mounjaro off-label to treat obesity in the wake of semaglutide (Wegovy) shortages and rising rates of obesity. The increased off-label use no doubt contributed to the reported $1.4 billion Mounjaro sales in the third quarter of 2023, up from $187.3 million during the same period of 2022. Based on projected sales potential for Zepbound, the company expects to topple those numbers for 2024’s near-term earnings.
Mechanism
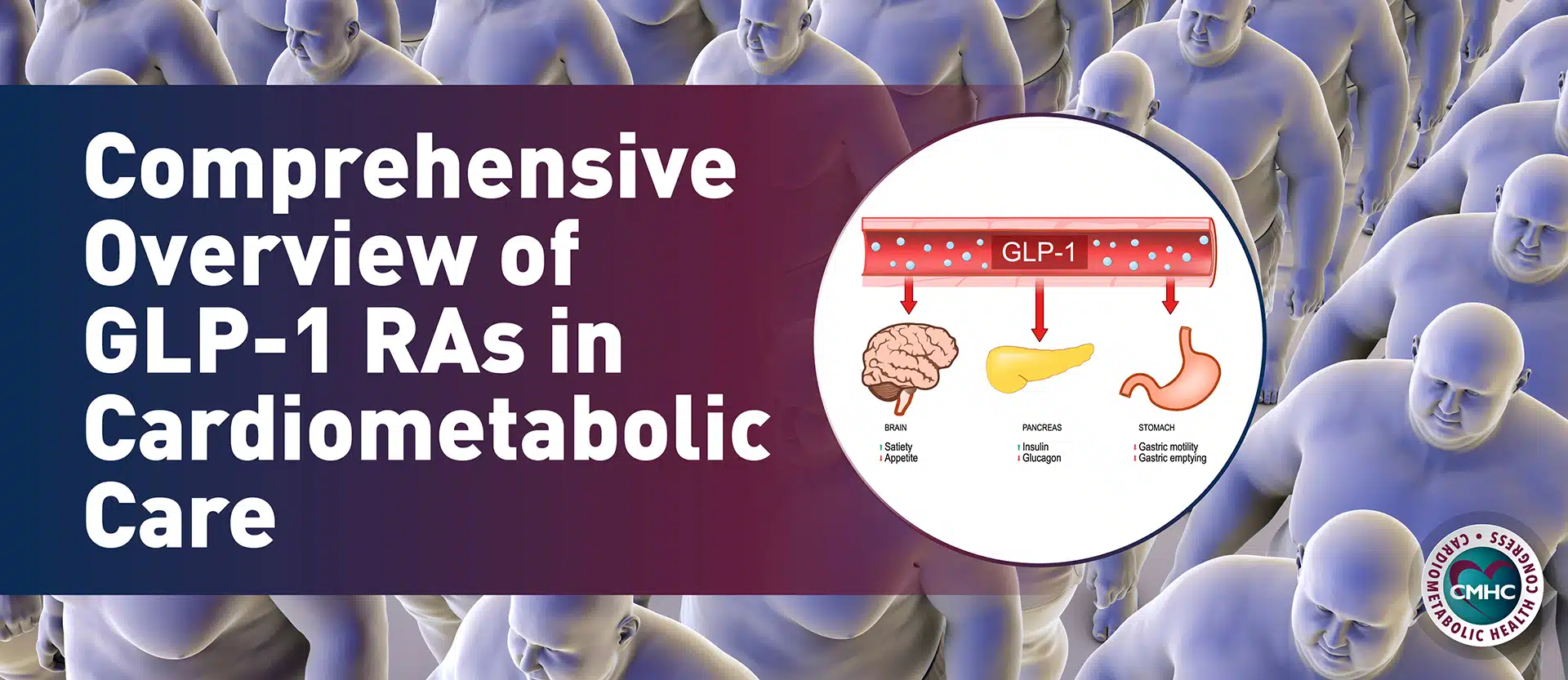
Tirzepatide activates glucagon-like peptide-1 (GLP-1), a hormone that helps reduce food intake and appetite. But unlike Novo Nordisk’s formulations of semaglutide (Wegovy and Ozempic), Zepbound also imitates the glucose-dependent insulinotropic polypeptide (GIP) hormone which can improve sugar and fat metabolism in addition to reducing hunger. Zepbound’s new FDA label instructs it be injected under the skin once weekly, and that the dosage must be “increased over four to 20 weeks to achieve the target dosages of 5 milligram (mg), 10 mg or 15 mg once weekly.” The maximum dosage of Zepbound is 15 mg.
Evidence
Zepbound’s effectiveness for chronic weight management was demonstrated in the phase 3 SURMOUNT-1 and SURMOUNT-2 trials. After 72 weeks of treatment, patients who received tirzepatide (Zepbound) experienced an average reduction in body weight of 18%, which was significantly higher than those who received a placebo. Some trial participants experienced gastrointestinal side effects such as “nausea, diarrhea, vomiting, constipation, abdominal (stomach) discomfort and pain, injection site reactions, fatigue, hypersensitivity (allergic) reactions (typically fever and rash), burping, hair loss and gastroesophageal reflux disease.” These side effects were generally mild and similar to those observed with other GLP-1s during clinical trials.
Affordability
Despite saying its newest agent will be about 20% less expensive than its competitors, Lilly projects the out-of-pocket cost for a month’s supply of Zepbound will be around $1,060 when it becomes available in the U.S. That is still a hefty price tag for patients who do not have insurance coverage to offset the cost of the drug. According to Mike Mason, president of Eli Lilly’s diabetes and obesity unit, those with commercial insurance may be eligible to pay as low as $25 per month if their carrier covers Zepbound, or about $550 per month if the drug isn’t covered by their policy.
Key takeaway
The approval of Zepbound comes at an unprecedented time for the community of obesity medicine providers concerned with helping patients lose weight. A months-long supply shortage of Novo Nordisk’s Wegovy already had providers scrambling to prescribe Ozempic and Mounjaro off-label for weight loss, or looking to older agents like liraglutide (Saxenda or Victoza) to meet the demand for GLP-1s. A new agent on the market may ease the stress on both patients and prescribers to secure access to weight loss agents that can improve cardiometabolic health and prevent obesity-related complications. Providers who treat children and adolescents with obesity are likely hoping the FDA will expand Zepbound’s label to include pediatric use, as it did for Wegovy in Dec. 2022.
Source
- FDA approves new medication for chronic weight management (2023) U.S. Food and Drug Administration. FDA. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-new-medication-chronic-weight-management (Accessed: November 8, 2023).