Exciting outcomes from the STEP TEENS trial were presented at ObesityWeek 2022 and simultaneously published in the New England Journal of Medicine. The outcomes of the phase 3 study indicate that adolescents with obesity benefit from once-weekly injection semaglutide over placebo.
With over 100 sessions, ObesityWeek 2022 is the largest international conference on obesity, coinciding with the 40th anniversary of the Obesity Society. In one lively presentation at this year’s conference, the results of the STEP TEENS study were discussed by its authors and the field’s foremost experts in pediatric obesity. The drug’s success in treating adults in clinical trials and subsequent 2021 approval made the STEP TEENS results highly anticipated, and favorable results were expected. However, there are challenges in the delivery of the drug that are likely to be compounded if its label is expanded to include adolescents.
STEP TEENS study design and outcomes
To analyze semaglutide in adolescents, investigators conducted a double-blind, parallel-group, randomized, placebo-controlled trial. They enrolled 201 adolescents between 12 and 18 years old with obesity or overweight to receive either once-weekly subcutaneous injections of semaglutide 2.4 mg or placebo. All received concurrent lifestyle intervention, which was defined as “counseling on healthy nutrition and physical activity,” throughout the trial period.
At the end of the 68-week study period, the participants assigned to semaglutide had an average 16.1% decrease in BMI, while the BMI of those in the placebo group actually increased by an average of 0.6%. Another metric of the results shows that 72.5% of semaglutide participants had achieved at least 5% weight loss compared to just 17% of those on placebo.
The outcomes analysis also showed improvements in risk factors such as waist circumference, blood glucose control, low and very low-density lipoprotein cholesterol, triglycerides, and liver enzymes compared with the placebo group. However, there was no statistically significant difference in blood pressure or high-density lipoprotein cholesterol between the study and placebo groups.
According to senior author Silva Arslanian, M.D., professor of pediatrics and clinical and translational science at the University of Pittsburgh School of Medicine, “The results are amazing. For a person who is 5’5” tall and weighs 240 pounds, the average reduction in BMI equates to shedding about 40 pounds.”
Semaglutide was approved for adults in 2021
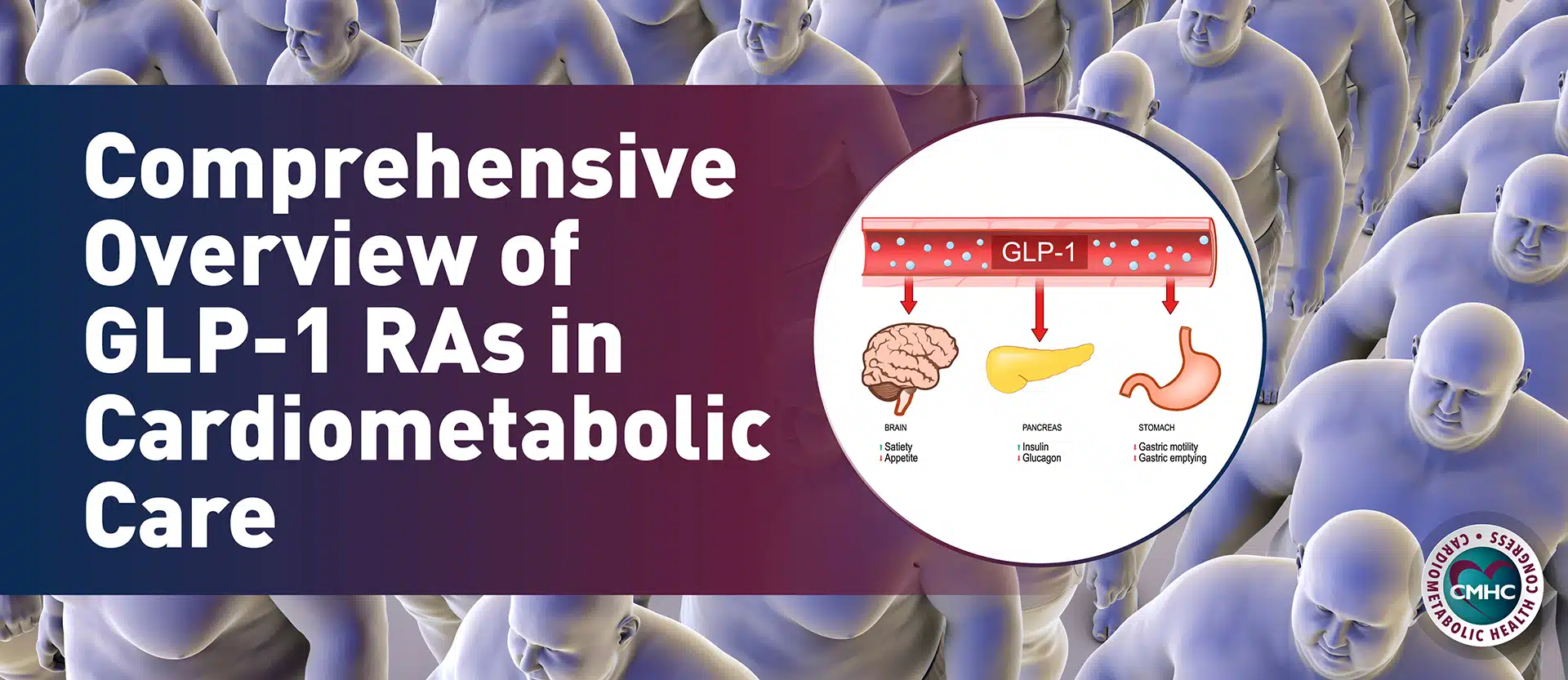
Semaglutide, sold by Novo Nordisk under the brand name Wegovy, mimics glucagon-like peptide-1 (GLP-1) to target areas of the brain that decrease hunger and improve appetite control. In 2021, this 
Obesity in adolescence
Worldwide, almost one in five children and adolescents have overweight or obesity. The disease is linked with decreased life expectancy and a higher risk of developing serious health problems such as type 2 diabetes, heart disease, nonalcoholic fatty liver disease, sleep apnea, and certain cancers. Depression, anxiety, poor self-esteem, and other psychological issues are also commonly found in teens with obesity. Currently, the only weight-loss drugs FDA-approved for use in adolescents ages 12 and older are liraglutide (Saxenda), orlistat (Xenical, Alli), and phentermine-topiramate (Qsymia); the STEP TEENS results suggest that semaglutide can outperform all of these agents.
When asked about potential negative impacts on developing adolescents, there was no hesitation from lead study author Daniel Weghuber, M.D., from the Department of Pediatrics at Paracelsus Medical University in Salzburg, Austria. “I’m really not afraid of a 15-year-old with 107 kg losing 10%, 15%, 20%” of their weight,” he said. While there is no indication of problems regarding normal growth or development in the study results, participants will be followed long-term to assess if adverse effects develop later in adulthood.
Future guidelines and challenges
There is little debate among clinicians that semaglutide will be included in the new American Academy of Pediatrics Section on Obesity’s clinical practice guidelines, which will also be discussed at ObesityWeek 2022. One challenge will be for these results to sway the thousands of providers who are hesitant to prescribe injection agents to adolescents with obesity. But, thousands of abstracts presented at ObesityWeek 2022 demonstrate that “watchful waiting” is not an effective strategy to manage obesity in the pediatric population. Another roadblock, reported by study funder and semaglutide manufacturer Novo Nordisk, is the ability to provide the drug to patients as a prolonged shortage continues. And many insurers (notably Medicare) refuse to cover the drug, which costs more than $1,300 a month out-of-pocket.
Key takeaway
Adolescents who received weekly doses of semaglutide 2.4 mg in conjunction with lifestyle intervention saw a greater reduction in BMI after 15 months than those given placebo plus lifestyle intervention, according to the STEP TEENS study. These results are significant because obesity in pediatric patients is alarmingly prevalent, but clinicians still underutilize drug therapies and bariatric surgery to treat it. However, the high cost and scarcity of semaglutide must be overcome with public health and other systems reform before this agent can be widely prescribed and its benefit can be seen on a larger scale.
“Obesity is not an issue of lack of willpower. That’s a major misunderstanding.” — Dr. Weghuber
UPDATE: On Jan. 3, 2023, the FDA approved the use of semaglutide in teens with obesity based on the results of this study. Read more here:
For more on treating obesity throughout the lifespan using the best therapies and informed strategies, join CMHC in Orlando for Taming the Flames: Inflammation in Cardiometabolic Disease